|
||||||
|
 | |
 | |
 | |
 | Open reading frame (ORF) annotation in the exon skipping event |
 | |
 | 3'-UTR located exon skipping events lost miRNA binding sites |
 | |
 | |
 | Splicing Quantitative Trait Loci (sQTLs) in the skipped exons |
 | |
 | |
 |
Gene summary for SNCA |
 Gene summary Gene summary |
| Gene information | Gene symbol | SNCA | Gene ID | 6622 |
| Gene name | synuclein alpha | |
| Synonyms | NACP|PARK1|PARK4|PD1 | |
| Cytomap | 4q22.1 | |
| Type of gene | protein-coding | |
| Description | alpha-synucleinI+/--synucleinnon A-beta component of AD amyloidsynuclein alpha-140synuclein, alpha (non A4 component of amyloid precursor)truncated alpha synuclein | |
| Modification date | 20200329 | |
| UniProtAcc | ||
| Context | - 27184464(SNCA Gene Polymorphism May Contribute to an Increased Risk of Alzheimer's Disease) |
 Gene ontology of each this gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each this gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Gene | GO ID | GO term | PubMed ID |
| SNCA | GO:0001921 | positive regulation of receptor recycling | 18980610 |
| SNCA | GO:0006919 | activation of cysteine-type endopeptidase activity involved in apoptotic process | 21050448 |
| SNCA | GO:0010040 | response to iron(II) ion | 11850416 |
| SNCA | GO:0010517 | regulation of phospholipase activity | 15641770 |
| SNCA | GO:0010642 | negative regulation of platelet-derived growth factor receptor signaling pathway | 12239163 |
| SNCA | GO:0016079 | synaptic vesicle exocytosis | 28288128 |
| SNCA | GO:0031115 | negative regulation of microtubule polymerization | 21127069 |
| SNCA | GO:0031623 | receptor internalization | 18980610 |
| SNCA | GO:0031648 | protein destabilization | 21320589 |
| SNCA | GO:0032026 | response to magnesium ion | 11850416 |
| SNCA | GO:0032410 | negative regulation of transporter activity | 16882008 |
| SNCA | GO:0032496 | response to lipopolysaccharide | 12406186 |
| SNCA | GO:0032769 | negative regulation of monooxygenase activity | 11943812 |
| SNCA | GO:0034341 | response to interferon-gamma | 19157893 |
| SNCA | GO:0035067 | negative regulation of histone acetylation | 16959795 |
| SNCA | GO:0035493 | SNARE complex assembly | 20798282 |
| SNCA | GO:0035543 | positive regulation of SNARE complex assembly | 20798282 |
| SNCA | GO:0045807 | positive regulation of endocytosis | 18980610 |
| SNCA | GO:0050729 | positive regulation of inflammatory response | 25533483 |
| SNCA | GO:0051262 | protein tetramerization | 21841800 |
| SNCA | GO:0051281 | positive regulation of release of sequestered calcium ion into cytosol | 15641770 |
| SNCA | GO:0051585 | negative regulation of dopamine uptake involved in synaptic transmission | 12958153 |
| SNCA | GO:0051612 | negative regulation of serotonin uptake | 16882008 |
| SNCA | GO:0051622 | negative regulation of norepinephrine uptake | 17156375 |
| SNCA | GO:0055074 | calcium ion homeostasis | 12239163 |
| SNCA | GO:0055114 | oxidation-reduction process | 21320589 |
| SNCA | GO:0060732 | positive regulation of inositol phosphate biosynthetic process | 15641770 |
| SNCA | GO:0070495 | negative regulation of thrombin-activated receptor signaling pathway | 12239163 |
| SNCA | GO:0070555 | response to interleukin-1 | 12406186 |
| SNCA | GO:0071280 | cellular response to copper ion | 21320589 |
| SNCA | GO:0071902 | positive regulation of protein serine/threonine kinase activity | 21127069 |
| SNCA | GO:1901215 | negative regulation of neuron death | 15863497 |
| SNCA | GO:1901216 | positive regulation of neuron death | 25533483 |
| SNCA | GO:1903284 | positive regulation of glutathione peroxidase activity | 23507046 |
| SNCA | GO:1903285 | positive regulation of hydrogen peroxide catabolic process | 23507046 |
Top |
Gene structures and expression levels for SNCA |
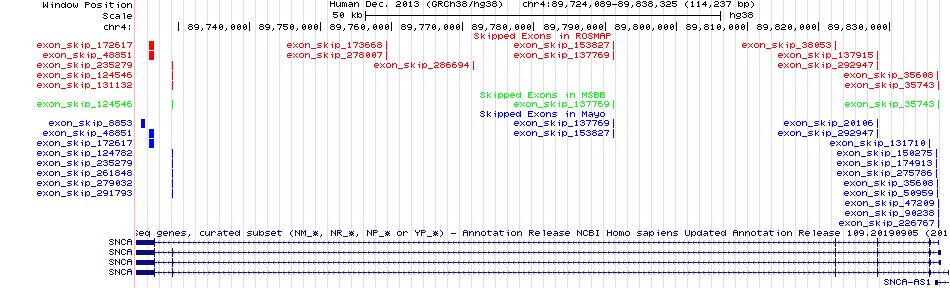
 Skipped exons in the ROSMAP, MSBB, and Mayo based on Ensembl gene isoform structure. Skipped exons in the ROSMAP, MSBB, and Mayo based on Ensembl gene isoform structure. * Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 |
ENSG00000145335
 Differentially expressed gene analysis across multiple brain tissues between AD and control. Differentially expressed gene analysis across multiple brain tissues between AD and control. |
| Tissue type | DEG direction | Base mean exp. | log2FC(AD/control) | P-value | Adj. p-value |
 Differentially expressed isoform analysis across multiple brain tissues between AD and control. Differentially expressed isoform analysis across multiple brain tissues between AD and control. |
| Tissue type | DEG direction | ENST | Transcript info. | Base mean exp. | log2FC(AD/control) | P-value | Adjc. p-value |
| STG | UP | ENST00000394991.7 | SNCA-205:protein_coding:SNCA | 8.863219e+01 | 2.272956e+00 | 5.569282e-04 | 2.836766e-02 |
 Landscape of isoform expressions across multiple brain tissues between AD and control. Landscape of isoform expressions across multiple brain tissues between AD and control. |
Top |
Exon skipping events with PSIs in ROSMAP, MSBB, and Mayo for SNCA |
 Landscape of individual exon skipping event across AD tissues and controls (PSI heatmap). Landscape of individual exon skipping event across AD tissues and controls (PSI heatmap). |
 All exon skipping events in AD cohorts. All exon skipping events in AD cohorts. |
| Exon skip ID | chr | Exons involved in exon skipping | Skipped exon |
| exon_skip_124546 | chr4 | 89726628:89726660:89729194:89729277:89822246:89822388 | 89729194:89729277 |
| exon_skip_131710 | chr4 | 89828143:89828184:89835547:89835692:89836743:89836789 | 89835547:89835692 |
| exon_skip_137915 | chr4 | 89822246:89822388:89828143:89828184:89835547:89835667 | 89828143:89828184 |
| exon_skip_20106 | chr4 | 89822373:89822388:89828143:89828184:89835547:89835667 | 89828143:89828184 |
| exon_skip_261848 | chr4 | 89726628:89726660:89729194:89729277:89822246:89822343 | 89729194:89729277 |
| exon_skip_291793 | chr4 | 89726628:89726660:89729194:89729277:89822246:89822289 | 89729194:89729277 |
| exon_skip_292947 | chr4 | 89822352:89822388:89828143:89828184:89835547:89835667 | 89828143:89828184 |
| exon_skip_35743 | chr4 | 89835547:89835692:89836743:89836789:89836962:89837076 | 89836743:89836789 |
| exon_skip_38053 | chr4 | 89729194:89729277:89822246:89822388:89835547:89835667 | 89822246:89822388 |
| exon_skip_47209 | chr4 | 89835655:89835692:89836743:89836789:89836962:89837076 | 89836743:89836789 |
| exon_skip_90238 | chr4 | 89835671:89835692:89836743:89836789:89836962:89837076 | 89836743:89836789 |
 Differentially expressed PSI values of individual exon skipping events in multiple brain tissues between AD and control. Differentially expressed PSI values of individual exon skipping events in multiple brain tissues between AD and control. |
| Exon skipping information | Tissue type | Avg(PSIs) in AD | Avg(PSIs) in control | Difference (PSI) | Adj. p-value |
Top |
Open reading frame (ORF) annotation in the exon skipping event for SNCA |
 Open reading frame (ORF) of individual exon skipping events in ROSMAP based on the Ensembl gene structure combined from isoforms. Open reading frame (ORF) of individual exon skipping events in ROSMAP based on the Ensembl gene structure combined from isoforms. |
| ENST | Start of skipped exon | End of skipped exon | ORF |
| ENST00000336904 | 89729194 | 89729277 | In-frame |
| ENST00000394986 | 89729194 | 89729277 | In-frame |
| ENST00000394991 | 89729194 | 89729277 | In-frame |
| ENST00000506244 | 89729194 | 89729277 | In-frame |
| ENST00000508895 | 89729194 | 89729277 | In-frame |
| ENST00000336904 | 89828143 | 89828184 | In-frame |
| ENST00000394986 | 89828143 | 89828184 | In-frame |
| ENST00000394991 | 89828143 | 89828184 | In-frame |
| ENST00000506244 | 89828143 | 89828184 | In-frame |
| ENST00000508895 | 89828143 | 89828184 | In-frame |
 Open reading frame (ORF) of individual exon skipping events in MSBB based on the Ensembl gene structure combined from isoforms. Open reading frame (ORF) of individual exon skipping events in MSBB based on the Ensembl gene structure combined from isoforms. |
| ENST | Start of skipped exon | End of skipped exon | ORF |
| ENST00000336904 | 89729194 | 89729277 | In-frame |
| ENST00000394986 | 89729194 | 89729277 | In-frame |
| ENST00000394991 | 89729194 | 89729277 | In-frame |
| ENST00000506244 | 89729194 | 89729277 | In-frame |
| ENST00000508895 | 89729194 | 89729277 | In-frame |
 Open reading frame (ORF) of individual exon skipping events in Mayo based on the Ensembl gene structure combined from isoforms. Open reading frame (ORF) of individual exon skipping events in Mayo based on the Ensembl gene structure combined from isoforms. |
| ENST | Start of skipped exon | End of skipped exon | ORF |
| ENST00000394986 | 89835547 | 89835692 | 3UTR-3CDS |
| ENST00000336904 | 89729194 | 89729277 | In-frame |
| ENST00000394986 | 89729194 | 89729277 | In-frame |
| ENST00000394991 | 89729194 | 89729277 | In-frame |
| ENST00000506244 | 89729194 | 89729277 | In-frame |
| ENST00000508895 | 89729194 | 89729277 | In-frame |
| ENST00000336904 | 89828143 | 89828184 | In-frame |
| ENST00000394986 | 89828143 | 89828184 | In-frame |
| ENST00000394991 | 89828143 | 89828184 | In-frame |
| ENST00000506244 | 89828143 | 89828184 | In-frame |
| ENST00000508895 | 89828143 | 89828184 | In-frame |
Top |
Infer the effects of exon skipping event on protein functional features for SNCA |
p-ENSG00000145335_img4.png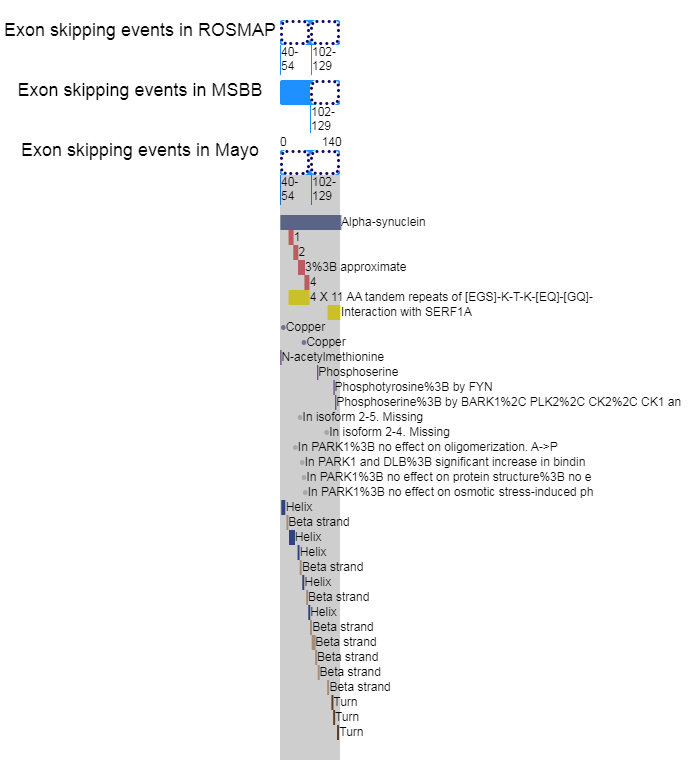 |
 Loci of skipped exons in ROSMAP across genomic, transcript, and protein sequence levels of In-frame cases. Loci of skipped exons in ROSMAP across genomic, transcript, and protein sequence levels of In-frame cases. |
| ENST | Length of mRNA | Length of AA seq. | Genomic start | Genomic end | mRNA start | mRNA end | AA start | AA end |
| ENST00000336904 | 3058 | 140 | 89828143 | 89828184 | 211 | 251 | 40 | 54 |
| ENST00000394986 | 1437 | 140 | 89828143 | 89828184 | 544 | 584 | 40 | 54 |
| ENST00000394991 | 1307 | 140 | 89828143 | 89828184 | 414 | 454 | 40 | 54 |
| ENST00000506244 | 587 | 140 | 89828143 | 89828184 | 218 | 258 | 40 | 54 |
| ENST00000508895 | 922 | 140 | 89828143 | 89828184 | 234 | 274 | 40 | 54 |
| ENST00000336904 | 3058 | 140 | 89729194 | 89729277 | 396 | 478 | 102 | 129 |
| ENST00000394986 | 1437 | 140 | 89729194 | 89729277 | 729 | 811 | 102 | 129 |
| ENST00000394991 | 1307 | 140 | 89729194 | 89729277 | 599 | 681 | 102 | 129 |
| ENST00000506244 | 587 | 140 | 89729194 | 89729277 | 403 | 485 | 102 | 129 |
| ENST00000508895 | 922 | 140 | 89729194 | 89729277 | 419 | 501 | 102 | 129 |
 Loci of skipped exons in MSBB across genomic, transcript, and protein sequence levels of In-frame cases. Loci of skipped exons in MSBB across genomic, transcript, and protein sequence levels of In-frame cases. |
| ENST | Length of mRNA | Length of AA seq. | Genomic start | Genomic end | mRNA start | mRNA end | AA start | AA end |
| ENST00000336904 | 3058 | 140 | 89729194 | 89729277 | 396 | 478 | 102 | 129 |
| ENST00000394986 | 1437 | 140 | 89729194 | 89729277 | 729 | 811 | 102 | 129 |
| ENST00000394991 | 1307 | 140 | 89729194 | 89729277 | 599 | 681 | 102 | 129 |
| ENST00000506244 | 587 | 140 | 89729194 | 89729277 | 403 | 485 | 102 | 129 |
| ENST00000508895 | 922 | 140 | 89729194 | 89729277 | 419 | 501 | 102 | 129 |
 Loci of skipped exons in Mayo across genomic, transcript, and protein sequence levels of In-frame cases. Loci of skipped exons in Mayo across genomic, transcript, and protein sequence levels of In-frame cases. |
| ENST | Length of mRNA | Length of AA seq. | Genomic start | Genomic end | mRNA start | mRNA end | AA start | AA end |
| ENST00000336904 | 3058 | 140 | 89828143 | 89828184 | 211 | 251 | 40 | 54 |
| ENST00000394986 | 1437 | 140 | 89828143 | 89828184 | 544 | 584 | 40 | 54 |
| ENST00000394991 | 1307 | 140 | 89828143 | 89828184 | 414 | 454 | 40 | 54 |
| ENST00000506244 | 587 | 140 | 89828143 | 89828184 | 218 | 258 | 40 | 54 |
| ENST00000508895 | 922 | 140 | 89828143 | 89828184 | 234 | 274 | 40 | 54 |
| ENST00000336904 | 3058 | 140 | 89729194 | 89729277 | 396 | 478 | 102 | 129 |
| ENST00000394986 | 1437 | 140 | 89729194 | 89729277 | 729 | 811 | 102 | 129 |
| ENST00000394991 | 1307 | 140 | 89729194 | 89729277 | 599 | 681 | 102 | 129 |
| ENST00000506244 | 587 | 140 | 89729194 | 89729277 | 403 | 485 | 102 | 129 |
| ENST00000508895 | 922 | 140 | 89729194 | 89729277 | 419 | 501 | 102 | 129 |
 Lost protein functional features of individual exon skipping events in ROSMAP. Lost protein functional features of individual exon skipping events in ROSMAP. |
| UniProt acc. | Start of exon skipping (AA) | End of exon skipping (AA) | Protein feature start (AA) | Protein feature end (AA) | Category of protein feature | Description of feature |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
 Lost protein functional features of individual exon skipping events in MSBB. Lost protein functional features of individual exon skipping events in MSBB. |
| UniProt acc. | Start of exon skipping (AA) | End of exon skipping (AA) | Protein feature start (AA) | Protein feature end (AA) | Category of protein feature | Description of feature |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
 Lost protein functional features of individual exon skipping events in Mayo. Lost protein functional features of individual exon skipping events in Mayo. |
| UniProt acc. | Start of exon skipping (AA) | End of exon skipping (AA) | Protein feature start (AA) | Protein feature end (AA) | Category of protein feature | Description of feature |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 41 | 54 | Alternative sequence | ID=VSP_006363;Note=In isoform 2-5. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:7601450;Dbxref=PMID:7601450 |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 45 | 47 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4BXL |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 41 | 44 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 52 | 55 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:3Q27 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Metal binding | Note=Copper;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 50 | 50 | Mutagenesis | Note=Impairs copper-binding. H->A;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21319811;Dbxref=PMID:21319811 |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 46 | 46 | Natural variant | ID=VAR_022703;Note=In PARK1 and DLB%3B significant increase in binding to negatively charged phospholipid liposomes%3B increases oligomerization. E->K;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:14755719,ECO:0000269|PubMe |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 50 | 50 | Natural variant | ID=VAR_070171;Note=In PARK1%3B no effect on protein structure%3B no effect on phosphorylation of the protein%3B no effect on membrane- and lipid-binding%3B increases oligomerization%3B increases fibril formation%3B increases secretion of the protein%3B im |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 53 | 53 | Natural variant | ID=VAR_007454;Note=In PARK1%3B no effect on osmotic stress-induced phosphorylation%3B increases oligomerization. A->T;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:12893833,ECO:0000269|PubMed:25561023,ECO:0000269|PubMed:919 |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 20 | 67 | Region | Note=4 X 11 AA tandem repeats of [EGS]-K-T-K-[EQ]-[GQ]-V-X(4) |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 31 | 41 | Repeat | Note=2 |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 40 | 54 | 42 | 56 | Repeat | Note=3%3B approximate |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 103 | 130 | Alternative sequence | ID=VSP_006364;Note=In isoform 2-4. Missing;Ontology_term=ECO:0000303,ECO:0000303;evidence=ECO:0000303|PubMed:7601450,ECO:0000303|PubMed:7802671;Dbxref=PMID:7601450,PMID:7802671 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 110 | 113 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 1 | 140 | Chain | ID=PRO_0000184022;Note=Alpha-synuclein |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Modified residue | Note=Phosphotyrosine%3B by FYN;Ontology_term=ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:11162638,ECO:0000269|PubMed:12893833;Dbxref=PMID:11162638,PMID:12893833 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 129 | 129 | Modified residue | Note=Phosphoserine%3B by BARK1%2C PLK2%2C CK2%2C CK1 and GRK5;Ontology_term=ECO:0000269,ECO:0000269,ECO:0000269;evidence=ECO:0000269|PubMed:10617630,ECO:0000269|PubMed:11813001,ECO:0000269|PubMed:24936070;Dbxref=PMID:10617630,PMID:11813001,PMID:24936070 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 125 | 125 | Mutagenesis | Note=Abolishes osmotic stress-induced phosphorylation. Y->F;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12893833;Dbxref=PMID:12893833 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 120 | 122 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
| P37840 | 102 | 129 | 124 | 126 | Turn | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1XQ8 |
Top |
3'-UTR located exon skipping events that lost miRNA binding sites in SNCA |
 3'-UTR exon skipping evnets lost miRNA binding. 3'-UTR exon skipping evnets lost miRNA binding. |
| Tissue type | ENST | Exon skip start | Exon skip end | microRNA | Binding site by TargetScan | Binding type by TargetScan | Bdinding site by miRanda | Score of miRanda | Energy by miRanda |
Top |
SNVs in the skipped exons for SNCA |
 - Differential PSIs between mutated versus non-mutated samples. - Differential PSIs between mutated versus non-mutated samples. |
 - Depth of Coverage in the skipped exon of the mutated samples. - Depth of Coverage in the skipped exon of the mutated samples. |
 - Sashimi plot in the skipped exon of the mutated samples. - Sashimi plot in the skipped exon of the mutated samples. |
 - Non-synonymous mutations located in the skipped exons. - Non-synonymous mutations located in the skipped exons. |
| Cancer type | Sample | ESID | Skipped exon start | Skipped exon end | Mutation start | Mutation end | Mutation type | Reference seq | Mutation seq | AAchange |
 - Non-synonymous mutations located in the skipped exons in CCLE. - Non-synonymous mutations located in the skipped exons in CCLE. |
| Sample | Skipped exon start | Skipped exon end | Mutation start | Mutation end | Mutation type | Reference seq | Mutation seq | AAchange |
Top |
AD stage-associated exon skippint events for SNCA |
 Associated exon skipping events with Braak staging or Clinical Dementia Rating (CDR). Associated exon skipping events with Braak staging or Clinical Dementia Rating (CDR). |
| AD stage info | Cohort | Tissue | SE id | Coefficient | P-value | Chromosome | Strand | E1 start | E1 end | Skipped start | Skipped end | E2 start | E2 end |
Top |
Splicing Quantitative Trait Loci (sQTL) in the exon skipping event for SNCA |
 sQTL information located at the skipped exons. sQTL information located at the skipped exons. |
| Tissue type | Exon skip ID | SNP id | Location | P-value | FDR |
Top |
Correlation with RNA binding proteins (RBPs) for SNCA |
 Correlated RBP and related information. Correlated RBP and related information. |
| Tissue type | RBP name | Exon skip ID | Correlation coeifficient | P-value |
Top |
RelatedDrugs for SNCA |
 Approved drugs targeting this gene. Approved drugs targeting this gene. (DrugBank Version 5.1.0 2018-04-02) |
| UniProtAcc | DrugBank ID | Drug name | Drug activity | Drug type | Drug status |
Top |
RelatedDiseases for SNCA |
 Diseases associated with this gene. Diseases associated with this gene. (DisGeNet 4.0) |
| Gene | Disease ID | Disease name | # pubmeds | Source |
