| UniProt acc. | Start of exon skipping (AA) | End of exon skipping (AA) | Protein feature start (AA) | Protein feature end (AA) | Category of protein feature | Description of feature |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| UniProt acc. | Start of exon skipping (AA) | End of exon skipping (AA) | Protein feature start (AA) | Protein feature end (AA) | Category of protein feature | Description of feature |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 1 | 62 | Alternative sequence | ID=VSP_056180;Note=In isoform 2. Missing;Ontology_term=ECO:0000303;evidence=ECO:0000303|PubMed:14702039;Dbxref=PMID:14702039 |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 6 | 15 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 17 | 19 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 22 | 30 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 33 | 40 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 52 | 56 | Beta strand | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 53 | 53 | Binding site | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 5 | 108 | Domain | Note=PH;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00145 |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 45 | 48 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:1UNQ |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Modified residue | Note=N6-acetyllysine;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 17 | 17 | Mutagenesis | Note=No effect on membrane localization. Loss of membrane localization%3B when associated with Q-20. E->K;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Substantial reduction of phosphorylation at T-308 and S-473%2C reduced AKT activation%2C and reduced binding to PIP3 as well as IGF1-induced membrane recruitment. Loss of membrane localization%3B when associated with K-17. K->Q;Ontology_term=ECO:0000 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 20 | 20 | Mutagenesis | Note=Slight increase of phosphorylation at T-308 and S-473. K->R;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:21775285;Dbxref=PMID:21775285 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 25 | 25 | Mutagenesis | Note=Impairs interaction with PtdIns(3%2C4%2C5)P3 and PtdIns(3%2C4)P2. R->A%2CC;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:12176338;Dbxref=PMID:12176338 |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 17 | 17 | Natural variant | ID=VAR_055422;Note=In PROTEUSS and breast cancer%3B also detected in colorectal and ovarian cancer%3B somatic mutation%3B results in increased phosphorylation at T-308 and higher basal ubiquitination%3B the mutant protein is more efficiently recruited to |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 25 | 25 | Natural variant | ID=VAR_069791;Note=In CWS6. R->C;Ontology_term=ECO:0000269;evidence=ECO:0000269|PubMed:23246288;Dbxref=dbSNP:rs397514644,PMID:23246288 |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 14 | 19 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 15 | 58 | 23 | 25 | Region | Note=Inositol-(1%2C3%2C4%2C5)-tetrakisphosphate binding |
| P31749 | 234 | 275 | 274 | 274 | Active site | Note=Proton acceptor;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159,ECO:0000255|PROSITE-ProRule:PRU10027 |
| P31749 | 234 | 275 | 274 | 274 | Active site | Note=Proton acceptor;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159,ECO:0000255|PROSITE-ProRule:PRU10027 |
| P31749 | 234 | 275 | 274 | 274 | Active site | Note=Proton acceptor;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159,ECO:0000255|PROSITE-ProRule:PRU10027 |
| P31749 | 234 | 275 | 274 | 274 | Active site | Note=Proton acceptor;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159,ECO:0000255|PROSITE-ProRule:PRU10027 |
| P31749 | 234 | 275 | 274 | 274 | Active site | Note=Proton acceptor;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159,ECO:0000255|PROSITE-ProRule:PRU10027 |
| P31749 | 234 | 275 | 274 | 274 | Active site | Note=Proton acceptor;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159,ECO:0000255|PROSITE-ProRule:PRU10027 |
| P31749 | 234 | 275 | 234 | 234 | Binding site | Note=Inhibitor |
| P31749 | 234 | 275 | 234 | 234 | Binding site | Note=Inhibitor |
| P31749 | 234 | 275 | 234 | 234 | Binding site | Note=Inhibitor |
| P31749 | 234 | 275 | 234 | 234 | Binding site | Note=Inhibitor |
| P31749 | 234 | 275 | 234 | 234 | Binding site | Note=Inhibitor |
| P31749 | 234 | 275 | 234 | 234 | Binding site | Note=Inhibitor |
| P31749 | 234 | 275 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 234 | 275 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 234 | 275 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 234 | 275 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 234 | 275 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 234 | 275 | 1 | 480 | Chain | ID=PRO_0000085605;Note=RAC-alpha serine/threonine-protein kinase |
| P31749 | 234 | 275 | 150 | 408 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| P31749 | 234 | 275 | 150 | 408 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| P31749 | 234 | 275 | 150 | 408 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| P31749 | 234 | 275 | 150 | 408 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| P31749 | 234 | 275 | 150 | 408 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| P31749 | 234 | 275 | 150 | 408 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| P31749 | 234 | 275 | 235 | 242 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 235 | 242 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 235 | 242 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 235 | 242 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 235 | 242 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 235 | 242 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 247 | 268 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 247 | 268 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 247 | 268 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 247 | 268 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 247 | 268 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 247 | 268 | Helix | Ontology_term=ECO:0000244;evidence=ECO:0000244|PDB:4GV1 |
| P31749 | 234 | 275 | 246 | 246 | Sequence conflict | Note=S->A;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P31749 | 234 | 275 | 246 | 246 | Sequence conflict | Note=S->A;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P31749 | 234 | 275 | 246 | 246 | Sequence conflict | Note=S->A;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P31749 | 234 | 275 | 246 | 246 | Sequence conflict | Note=S->A;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P31749 | 234 | 275 | 246 | 246 | Sequence conflict | Note=S->A;Ontology_term=ECO:0000305;evidence=ECO:0000305 |
| P31749 | 234 | 275 | 246 | 246 | Sequence conflict | Note=S->A;Ontology_term=ECO:0000305;evidence=ECO:0000305 |












 Gene summary
Gene summary Gene ontology of each this gene with evidence of Inferred from Direct Assay (IDA) from Entrez
Gene ontology of each this gene with evidence of Inferred from Direct Assay (IDA) from Entrez  Skipped exons in the ROSMAP, MSBB, and Mayo based on Ensembl gene isoform structure.
Skipped exons in the ROSMAP, MSBB, and Mayo based on Ensembl gene isoform structure. 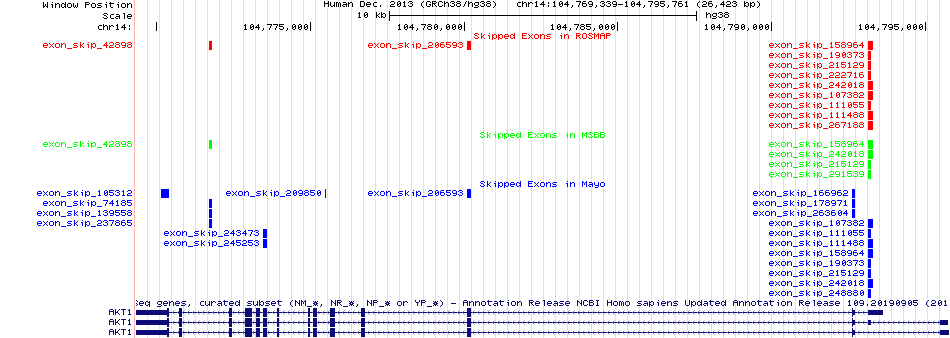
 Differentially expressed gene analysis across multiple brain tissues between AD and control.
Differentially expressed gene analysis across multiple brain tissues between AD and control. Differentially expressed isoform analysis across multiple brain tissues between AD and control.
Differentially expressed isoform analysis across multiple brain tissues between AD and control. Landscape of isoform expressions across multiple brain tissues between AD and control.
Landscape of isoform expressions across multiple brain tissues between AD and control. Landscape of individual exon skipping event across AD tissues and controls (PSI heatmap).
Landscape of individual exon skipping event across AD tissues and controls (PSI heatmap). All exon skipping events in AD cohorts.
All exon skipping events in AD cohorts. Differentially expressed PSI values of individual exon skipping events in multiple brain tissues between AD and control.
Differentially expressed PSI values of individual exon skipping events in multiple brain tissues between AD and control. Open reading frame (ORF) of individual exon skipping events in ROSMAP based on the Ensembl gene structure combined from isoforms.
Open reading frame (ORF) of individual exon skipping events in ROSMAP based on the Ensembl gene structure combined from isoforms. Open reading frame (ORF) of individual exon skipping events in MSBB based on the Ensembl gene structure combined from isoforms.
Open reading frame (ORF) of individual exon skipping events in MSBB based on the Ensembl gene structure combined from isoforms. Open reading frame (ORF) of individual exon skipping events in Mayo based on the Ensembl gene structure combined from isoforms.
Open reading frame (ORF) of individual exon skipping events in Mayo based on the Ensembl gene structure combined from isoforms.
 Loci of skipped exons in ROSMAP across genomic, transcript, and protein sequence levels of In-frame cases.
Loci of skipped exons in ROSMAP across genomic, transcript, and protein sequence levels of In-frame cases. Loci of skipped exons in MSBB across genomic, transcript, and protein sequence levels of In-frame cases.
Loci of skipped exons in MSBB across genomic, transcript, and protein sequence levels of In-frame cases. Loci of skipped exons in Mayo across genomic, transcript, and protein sequence levels of In-frame cases.
Loci of skipped exons in Mayo across genomic, transcript, and protein sequence levels of In-frame cases. Lost protein functional features of individual exon skipping events in ROSMAP.
Lost protein functional features of individual exon skipping events in ROSMAP. Lost protein functional features of individual exon skipping events in MSBB.
Lost protein functional features of individual exon skipping events in MSBB. Lost protein functional features of individual exon skipping events in Mayo.
Lost protein functional features of individual exon skipping events in Mayo. 3'-UTR exon skipping evnets lost miRNA binding.
3'-UTR exon skipping evnets lost miRNA binding. - Differential PSIs between mutated versus non-mutated samples.
- Differential PSIs between mutated versus non-mutated samples. - Depth of Coverage in the skipped exon of the mutated samples.
- Depth of Coverage in the skipped exon of the mutated samples. - Sashimi plot in the skipped exon of the mutated samples.
- Sashimi plot in the skipped exon of the mutated samples. - Non-synonymous mutations located in the skipped exons.
- Non-synonymous mutations located in the skipped exons. - Non-synonymous mutations located in the skipped exons in CCLE.
- Non-synonymous mutations located in the skipped exons in CCLE. Associated exon skipping events with Braak staging or Clinical Dementia Rating (CDR).
Associated exon skipping events with Braak staging or Clinical Dementia Rating (CDR). sQTL information located at the skipped exons.
sQTL information located at the skipped exons. Correlated RBP and related information.
Correlated RBP and related information. Approved drugs targeting this gene.
Approved drugs targeting this gene.  Diseases associated with this gene.
Diseases associated with this gene. 