
|
||||||
|
 | |
 | |
 | Expression of this gene in the resistant group across all datasets and cell types |
 | Significant ligand-receptor pairs related to this gene (This gene does not contain this module) |
 | Known drug resistance mechanisms of this gene (This gene does not contain this module) |
 | MicroRNAs (miRNAs) regulating this gene (This gene does not contain this module) |
 | Motifs and transcription factors (TFs) regulating this gene (This gene does not contain this module) |
 | Acts as a transcription factor (This gene does not contain this module) |
 | Acts as a drug target< (This gene does not contain this module) |
Gene: MT-ATP8 |
Summary for MT-ATP8 |
| Gene information | Gene symbol | MT-ATP8 | Ensembl ID | ENSG00000228253 |
| Entrez ID | 4509 | |
| Gene name | ATP synthase F0 subunit 8 | |
| Synonyms | A6L|ATP8|MTATP8|URFA6L | |
| Gene type | protein_coding | |
| UniProtAcc | P03928 |
Top |
Dataset with differentially expressed gene: MT-ATP8 |
 Differentially expression genes between the resistant and sensitive groups. A positive avg_log2FC represents significantly high expression in the resistant group, while a negative avg_log2FC represents significantly low expression in the resistant group. Differentially expression genes between the resistant and sensitive groups. A positive avg_log2FC represents significantly high expression in the resistant group, while a negative avg_log2FC represents significantly low expression in the resistant group. |
| Dataset | Source | Tissue | Cancer type level1 | Cancer type level2 | Drug type | Regimen | Timepoint | Cell type | avg_log2FC | p_val_adj |
GSE186960 | Panc1 cell line | Cell line | Pancreatic cancer | Pancreatic ductal adenocarcinoma (PDAC) | Chemotherapy | gemcitabine | NA | Malignant cells | -2.28015 | 0.00e+00 |
GSE163836 | FCIBC02 cell line | Cell line | Breast cancer | Inflammatory breast cancer | Chemotherapy | paclitaxel | NA | Malignant cells | 0.495796 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | cDCs | 1.70787 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | NK cells | 0.829787 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | Progenitors | 0.757504 | 3.31e-04 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | Plasma cells | 1.34014 | 2.65e-40 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | Mono/Macro | 0.266358 | 2.85e-11 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | CD8+ T cells | 0.89461 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | B cells | 1.12748 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | CD4+ T cells | 0.639199 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | NK cells | 0.673033 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | CD8+ T cells | 0.83469 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | B cells | 0.554177 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | Plasma cells | 0.618554 | 1.92e-05 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | cDCs | 1.3121 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | Mono/Macro | 0.291198 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | CD4+ T cells | 0.324883 | 0.00e+00 |
GSE169246_PacBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | pDCs | 0.998212 | 5.13e-09 |
GSE169246_PacTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | B cells | 0.471031 | 4.79e-17 |
GSE169246_PacTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | CD4+ T cells | 0.328429 | 0.00e+00 |
GSE169246_PacTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | post | cDCs | 0.391045 | 3.81e-05 |
GSE169246_PacTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Chemotherapy | paclitaxel | pre | Plasma cells | 0.493586 | 1.68e-08 |
GSE153697 | patients | Bone marrow aspirate | Acute lymphoblastic leukemia | Relapsed B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | anti-CD19 CAR-T | NA | Malignant cells | 0.338168 | 9.36e-21 |
GSE169246_PacAteBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | post | pDCs | -0.71409 | 9.75e-03 |
GSE169246_PacAteBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | post | CD4+ T cells | 0.326792 | 0.00e+00 |
GSE169246_PacAteBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | post | cDCs | -0.579935 | 1.39e-13 |
GSE169246_PacAteBlood | patients | Peripheral blood mononuclear cells | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | CD8+ T cells | 0.455004 | 0.00e+00 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | post | CD4+ T cells | -0.494677 | 0.00e+00 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | post | NK cells | -0.586306 | 2.85e-15 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | post | CD8+ T cells | -0.916932 | 0.00e+00 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | Plasma cells | 0.914344 | 1.25e-33 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | CD8+ T cells | 0.694465 | 0.00e+00 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | B cells | 0.515368 | 4.68e-36 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | NK cells | 0.744516 | 0.00e+00 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | CD4+ T cells | 0.733148 | 0.00e+00 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | Mono/Macro | 0.583111 | 1.72e-02 |
GSE169246_PacAteTissue | patients | Tumor tissue | Breast cancer | Advanced triple-negative breast cancer (TNBC) | Immunotherapy | atezolizumab + paclitaxel | pre | Mast cells | 0.90606 | 1.29e-03 |
GSE197268_Axi-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | axi-cel (CAR-T) | post | Mono/Macro | 0.30118 | 0.00e+00 |
GSE197268_Axi-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | axi-cel (CAR-T) | post | CD4+ T cells | -0.442762 | 0.00e+00 |
GSE197268_Axi-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | axi-cel (CAR-T) | post | cDCs | 0.583356 | 4.35e-11 |
GSE197268_Axi-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | axi-cel (CAR-T) | pre | CD4+ T cells | 0.343783 | 2.92e-21 |
GSE197268_Axi-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | axi-cel (CAR-T) | pre | CD8+ T cells | 0.281669 | 2.34e-38 |
PMID34715028 | patients | Tumor tissue | Kidney cancer | Clear cell renal cell carcinoma (ccRCC) | Immunotherapy | nivolumab | post | CD4+ T cells | 0.963767 | 0.00e+00 |
PMID34715028 | patients | Tumor tissue | Kidney cancer | Clear cell renal cell carcinoma (ccRCC) | Immunotherapy | nivolumab | post | CD8+ T cells | 0.534292 | 0.00e+00 |
GSE212217 | patients | Peripheral blood mononuclear cells | Endometrial cancer | Epigenetic MMRd endometrial carcinoma | Immunotherapy | pembrolizumab | post | CD8+ T cells | 0.45723 | 0.00e+00 |
GSE212217 | patients | Peripheral blood mononuclear cells | Endometrial cancer | Epigenetic MMRd endometrial carcinoma | Immunotherapy | pembrolizumab | post | NK cells | 0.267287 | 0.00e+00 |
GSE212217 | patients | Peripheral blood mononuclear cells | Endometrial cancer | Epigenetic MMRd endometrial carcinoma | Immunotherapy | pembrolizumab | post | CD4+ T cells | 0.372132 | 0.00e+00 |
GSE212217 | patients | Peripheral blood mononuclear cells | Endometrial cancer | Epigenetic MMRd endometrial carcinoma | Immunotherapy | pembrolizumab | post | pDCs | 0.466267 | 1.08e-07 |
GSE212217 | patients | Peripheral blood mononuclear cells | Endometrial cancer | Epigenetic MMRd endometrial carcinoma | Immunotherapy | pembrolizumab | pre | NK cells | -0.321421 | 3.16e-26 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | B cells | -0.423519 | 9.98e-05 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | Malignant cells | -0.610865 | 1.51e-05 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | Plasma cells | -1.08745 | 6.84e-14 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | pDCs | -1.08443 | 9.31e-03 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | CD8+ T cells | -0.812625 | 0.00e+00 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | CD4+ T cells | -0.769106 | 0.00e+00 |
GSE207422_Sin | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | sintilimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | Mono/Macro | -0.654838 | 1.49e-13 |
GSE197268_Tisa-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | tisa-cel (CAR-T) | post | NK cells | 0.278108 | 4.40e-32 |
GSE197268_Tisa-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | tisa-cel (CAR-T) | post | CD4+ T cells | -0.32596 | 0.00e+00 |
GSE197268_Tisa-cel | patients | Peripheral blood mononuclear cells | Acute lymphoblastic leukemia | Large B-cell acute lymphoblastic leukemia (B-ALL) | Immunotherapy | tisa-cel (CAR-T) | pre | Mono/Macro | -0.404185 | 0.00e+00 |
GSE207422_Tor | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | toripalimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | CD4+ T cells | 0.506839 | 6.01e-26 |
GSE207422_Tor | patients | Tumor tissue | Lung cancer | EGFR/ALK mutation negative non-small cell lung cancer (NSCLC) | Immunotherapy | toripalimab + carboplatin + (docetaxel or pemetrexed or emcitabine) | post | B cells | 0.963829 | 1.31e-11 |
GSE161195 | patients | Bone marrow aspirate | Multiple myeloma | Refractory multiple myeloma (MM) | Targeted therapy | DARA-KRD (daratumumab + carfilzomib + lenalidomide + dexamethasone) | post | Malignant cells | 0.460522 | 5.63e-26 |
GSE149214 | PC9 cell line | Cell line | Lung cancer | EGFR-mutated non-small cell lung cancer (NSCLC) | Targeted therapy | erlotinib | NA | Malignant cells | 0.463461 | 1.05e-27 |
GSE199333 | patients | Bone marrow aspirate | Acute myeloid leukemia | Primary FLT3-ITD-mutated acute myeloid leukemia (AML) | Targeted therapy | gilteritinib | post | Malignant cells | -0.325987 | 0.00e+00 |
GSE104987 | MCF7 cell line | Cell line | Breast cancer | ER+ breast cancer | Targeted therapy | KDM5-C70 | NA | Malignant cells | 0.895459 | 0.00e+00 |
GSE108397 | 451Lu cell line | Cell line | Melanoma | Melanoma | Targeted therapy | PLX-4720 | NA | Malignant cells | -0.40298 | 0.00e+00 |
GSE175716 | PDX | Tumor tissue | Liver cancer | Advanced hepatocellular carcinoma | Targeted therapy | sorafenib | NA | Malignant cells | 1.3866 | 0.00e+00 |
GSE115251 | Kelly cell line | Cell line | Neuroblastoma | Neuroblastoma | Targeted therapy | TAE684 | NA | Malignant cells | -0.68449 | 0.00e+00 |
Top |
Expression of MT-ATP8 in the resistant group across all datasets and cell types |
 Red dots represent significantly high expression in the resistant group, while blue dots represent significantly low expression in the resistant group. White dots represent that there is no significant difference in the expression of this gene between the resistant and sensitive groups. Blank represents that this cell type is not present in this dataset. (If the image exists, the user can click on it to enlarge it in a new window.) Red dots represent significantly high expression in the resistant group, while blue dots represent significantly low expression in the resistant group. White dots represent that there is no significant difference in the expression of this gene between the resistant and sensitive groups. Blank represents that this cell type is not present in this dataset. (If the image exists, the user can click on it to enlarge it in a new window.) |
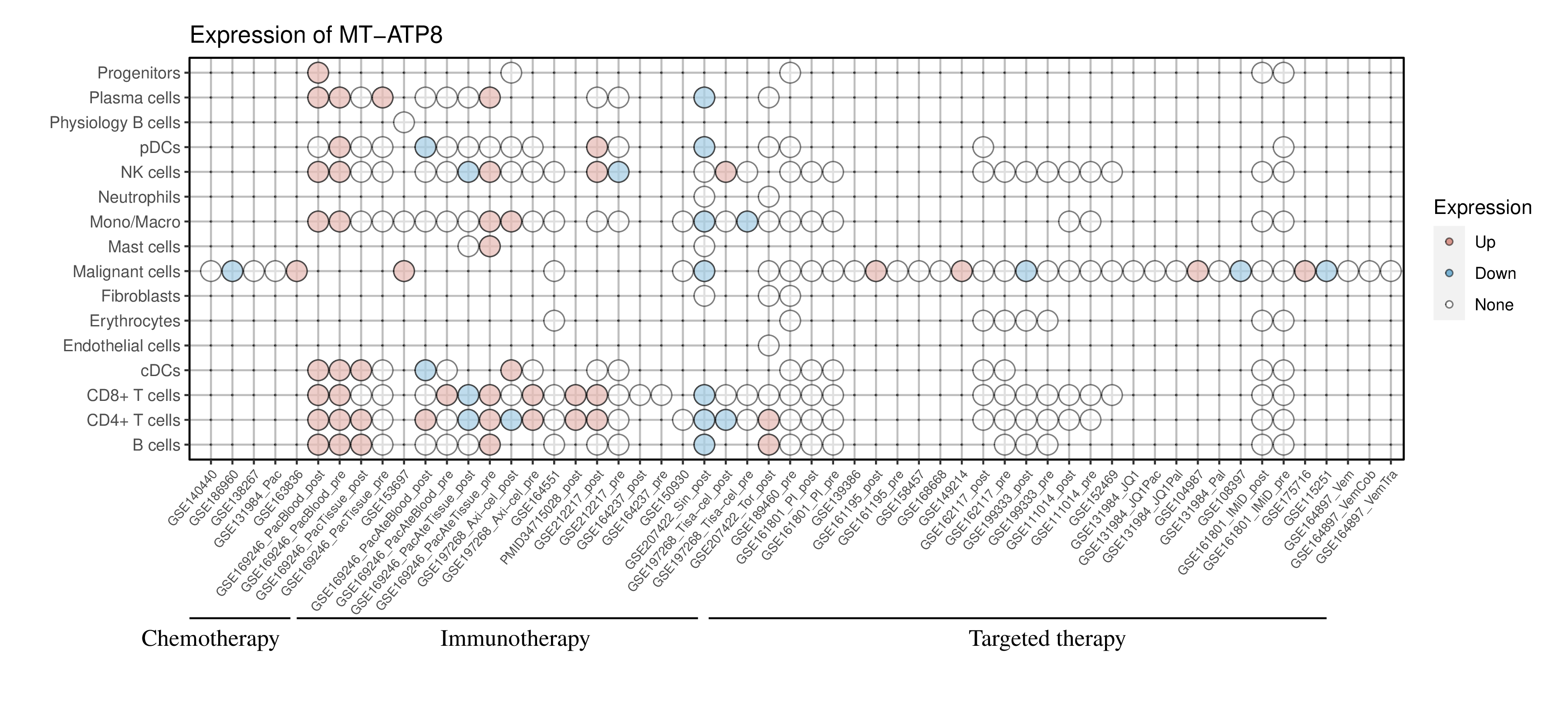 |
Top |
Significant ligand-receptor pairs related to MT-ATP8 |
 This table shows the significant ligand-receptor pairs related to this gene across all datasets, timepoints and conditions. (Complete files can be downloaded from the download section.) This table shows the significant ligand-receptor pairs related to this gene across all datasets, timepoints and conditions. (Complete files can be downloaded from the download section.) |
Top |
Known drug resistance mechanisms of this gene |
 This table shows the known drug resistance mechanisms of this gene. Clicking on this gene will link to another database that displays more drug resistance-related information. This table shows the known drug resistance mechanisms of this gene. Clicking on this gene will link to another database that displays more drug resistance-related information. |
Top |
MicroRNAs (miRNA) regulating MT-ATP8 |
 This table shows the miRNAs with a score of more than 80 regulating this gene. (Complete files can be downloaded from the download section.) This table shows the miRNAs with a score of more than 80 regulating this gene. (Complete files can be downloaded from the download section.) |
Top |
Motifs and transcription factors (TFs) regulating MT-ATP8 |
 This table shows the Motifs and transcription factors (TFs) regulating this gene. (Complete files can be downloaded from the download section.) This table shows the Motifs and transcription factors (TFs) regulating this gene. (Complete files can be downloaded from the download section.) |
Top |
Acts as a transcription factor |
 This table shows that this differential gene acts as a transcription factor. This table shows that this differential gene acts as a transcription factor. |
Top |
Acts as a drug target |
 This table shows that this differential gene acts as a drug target. This table shows that this differential gene acts as a drug target. |
| 1 | "Sun X, Zhang Y, Li H, Zhou Y, Shi S, Chen Z, He X, Zhang H, Li F, Yin J, Mou M, Wang Y, Qiu Y, Zhu F. DRESIS: the first comprehensive landscape of drug resistance information. Nucleic Acids Res. 2023 Jan 6;51(D1):D1263-D1275. doi: 10.1093/nar/gkac812. PMID: 36243960; PMCID: PMC9825618." |
| 2 | "Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. doi: 10.1093/nar/gkx1037. PMID: 29126136; PMCID: PMC5753335." |
